cross-posted from: https://slrpnk.net/post/3853502
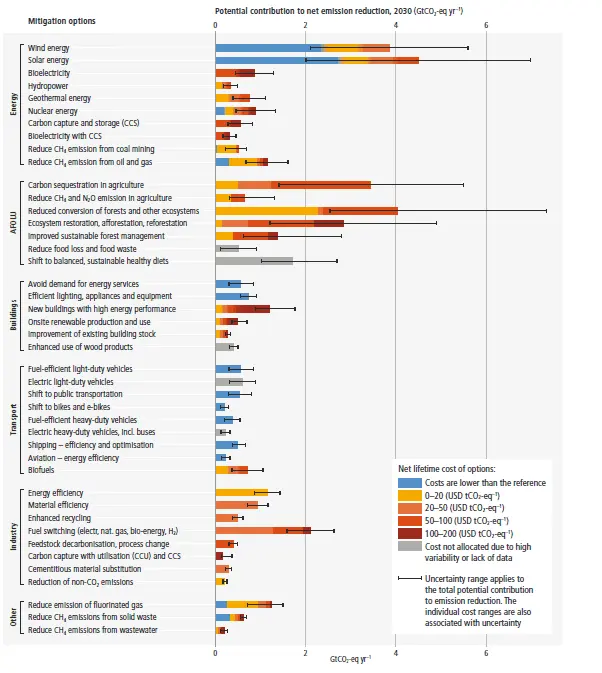
To be clear: carbon capture like this is a tiny and expensive part of what needs doing over the next few years
It’s getting a lot of support and publicity in large part because it’s backed by the oil industry, which is trying to create social permission for continued extraction and burning on a much larger scale than the removal.


From a simple standard heat of formation analysis of the calcination reaction (PDF), the minimum energy required to capture 1 metric ton of carbon dioxide using calcination is about 4 gigajoules. For the average US resident who generates 14.44 tons of carbon dioxide per year, the Heirloom technology would require approximately 19 kilowatts of energy per US resident to offset their carbon footprint. That’s equivalent to each person running 10 typical hair dryers continuously year-round.
From my experience studying chemical engineering, Heirloom’s process is an example of an “equilibrium-driven” process which relies upon chemical concentration gradients (CO₂-poor calcium oxide powder in trays passively absorbing comparatively CO₂-rich atmosphere) to drive the separation. In comparison, “pressure-driven” processes that exploit selective permeabilities of fluids through specialized membranes can be much more energy-efficient, provided high pressure piping and fluid compressors are available. That said, both strategies require a place to store the extracted CO₂ which likely involves additional energy costs such as raising the CO2 to supercritical pressure (around 200 bar) for injection into the earth.